Research PR showcase! Vol.2: Sex changes based on environmental factors
IRCAEB Dept. of Molecular Agriculture, Takeshi Kitano
IRCAEB research showcase Vol. 2 will feature research by Prof. Kitano about how the sex of fishes are influence by the environment.
Have you ever considered that there are two sexes in fishes like other animals? For instance, when you buy fishes from the supermarket
or see them in the aquarium, have you wondered if they’re male or female?
Unlike us humans, of which sexes are strictly determined by genetics, sex
determination in fishes are very flexible. In some fishes such as medaka
or flounders, genetically female individuals can become male (masculinize) when the water temperature is high. Considering such flexibility exists, what exactly is sex, and how did
it originate in evolution? We will explain the roles of genetics and environment
in this fascinating topic.
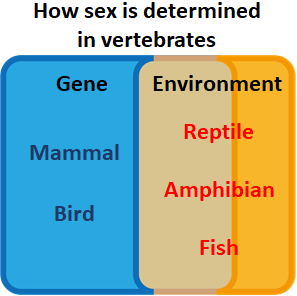
We humans are vertebrates, which means animals with backbones. Vertebrates
include other animals familiar to us such as mammals, birds, reptiles,
amphibians and fishes. For mammals and birds, sex is determined exclusively
by genetics (the combinations of sexual chromosomes). For reptiles and
amphibians, aside from genetics, egg incubation temperature can also change
the sexes of individuals. Fishes are the most flexible among vertebrates,
where even adults have been documented to change from females to males
depending on the environment. In another word, female fishes with ovaries
may eventually possess testes instead.
Above: It is thought that the –OH hydroxyl functional groups on graphene oxide help trap water molecules.
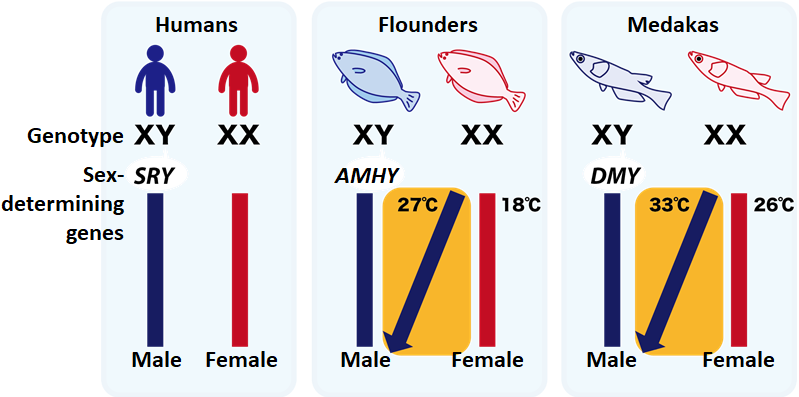
So how is sex determined by genetics in the first place? For humans, sex is determined by the combinations of sexual chromosomes, where males possess X and Y chromosomes while females possess two X chromosomes. A gene required for male sexual determination called SRYis found on the human Y chromosome. In another word, individuals with Y chromosomes but not functional SRY genes are still not physiologically male. Similarly for fishes, males are defined as individuals that possess Y chromosomes. However since fishes can change their sexes easily, for a long time it’s unclear whether fishes possess sex-determining genes similar to SRY. Thanks to the medaka research in Japan over the years, sex-determining gene has been identified in medaka in 2002. Recently, Prof. Kitano et al. also identified the sex-determining gene in flounders in 2022.
During the 1990s, environmental hormones made headlines all over Japan. Chemicals leaked into the environment (particularly to water bodies) were reported to cause female conch shells to develop male reproductive organs. Of course, their effects on fishes were also investigated, which brings us to Prof. Kitano’s medakaresearch.
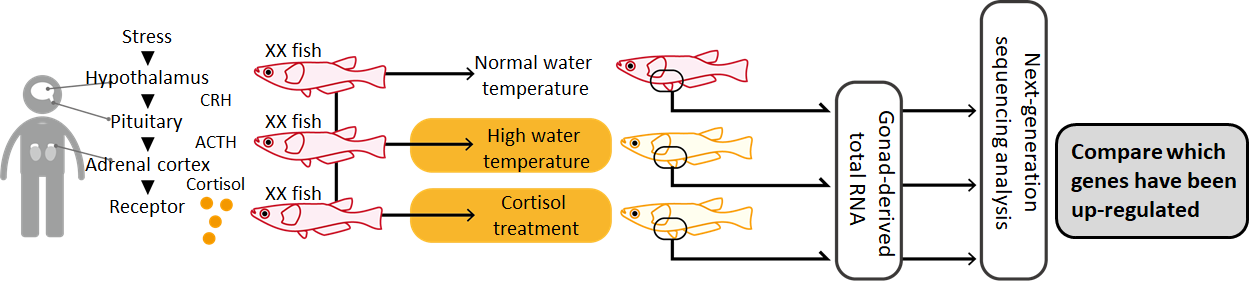
The shapes of medaka dorsal and pelvic fins are different between male and female, making sex identification relatively easy. In addition, mutant medakas with sexually dimorphic colorations have been developed over the years of medaka research in Japan. These traits make medaka a powerful experimental system for sexual development research. When raised in high temperature and high density, female medakas tend to masculinize. Actually this phenomenon is not limited to medaka, but is often observed in fish farms where fishes are kept in high density. It is not uncommon for male fishes to outnumber females in aquaculture.

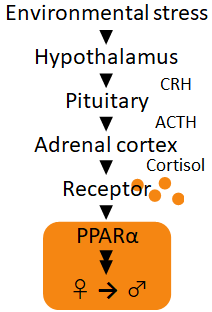
How does temperature and density change medaka’s body? First, these stimuli trigger the secretion of a hormone called cortisol, which is known to be active during stress. Cortisol then stimulates genetic changes associated with masculinization, and cause ovaries to differentiate into testes. Basically, cortisol causes changes in the gene functions of reproductive cells. As such, the transcriptomic changes of medaka gonads during cortisol stimulation were analyzed, to identify genes important for medaka masculinization.
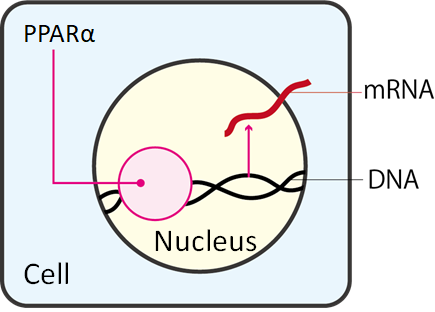
Interestingly, a gene called peroxisome proliferator-activated receptor α (PPARα) was found to be strongly up-regulated in masculinized medaka testes.
PPARα encodes a receptor protein that binds lipid molecules and trigger
transcriptional regulations, in order to regulate lipid metabolism. Other
researches have shown that PPARα is also activated during starvation, and
may play a role to inhibit senescence.
Due to the potential importance of PPARα in diabetes, cancer and schizophrenia, researches world-wide have led to the development of various PPARα agonists. We took advantage of these agonists to verify the roles of PPARα in medaka masculinization. Expectedly, 50 µM of PPARα agonist induced medaka masculinization with frequencies similar to cortisol treatment.
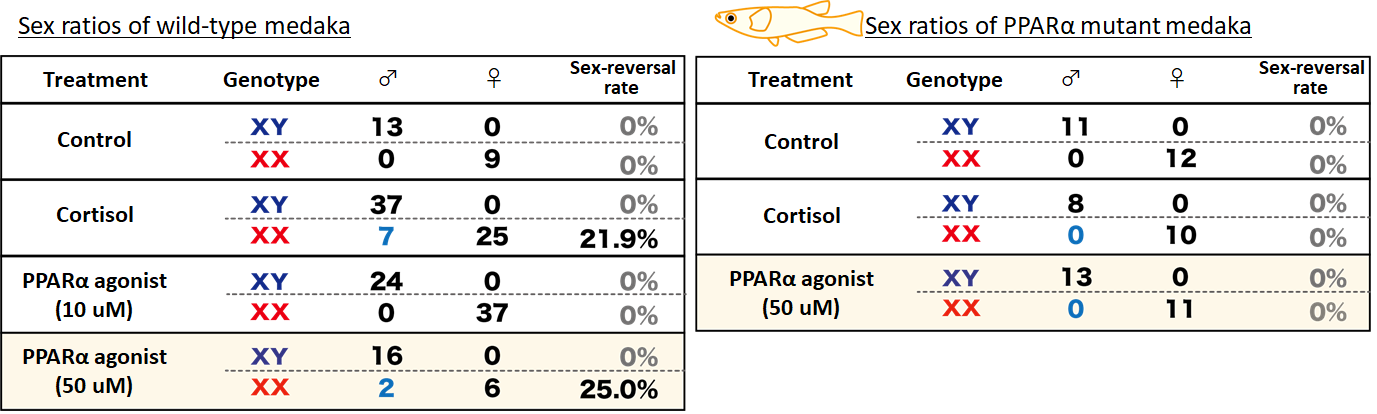
Similarly, we investigated what would happen if the endogenous PPARα gene
is mutated. We isolated PPARα-deficient medaka mutants, and checked their masculinization frequencies. Neither cortisol nor PPARα
agonist treatments were able to convert even a single PPARα mutant medaka
to male. Therefore PPARα is absolutely essential for masculinization during
high temperature and density.
The expression of PPARα was found to increase together with genes involved
with oxidative stress, which refer to the increase of reactive oxygen species (ROS) in the body. ROS naturally accumulate in all organisms, but since ROS
is harmful, most organisms possess mechanisms to eliminate ROS. Under normal
circumstances these mechanisms keep the ROS balance under control, but
when this balance is lost ROS accumulate and cause various problems for
the organism.
When medakas experience oxidative stress (induced by excess hydrogen peroxide
in the water), females masculinize as PPARα becomes activated, even in
the absence of cortisol. On the contrary, PPARα mutant medakas fail to
masculinize when placed under the same oxidative stress. Based on the results
of this experiment, cortisol-dependent masculinization induced by both
high temperature/density and oxidative stress require PPARα.
The importance of PPARα in human diseases have been investigated extensively.
Initially PPARα was discovered to be upregulated during starvation, PPARα
is now also known to regulate senescence. Since fishes are more evolutionarily
ancient than humans, the medaka masculinization “disease” may share similarities
with human diseases at the molecular level, even though humans do not masculinize
like medakas. Nevertheless, it is possible that masculinization evolved
independently in fishes, and is not conserved in other vertebrates. Either
way, the PPARα pathway may hold the keys to the origin of sexes.
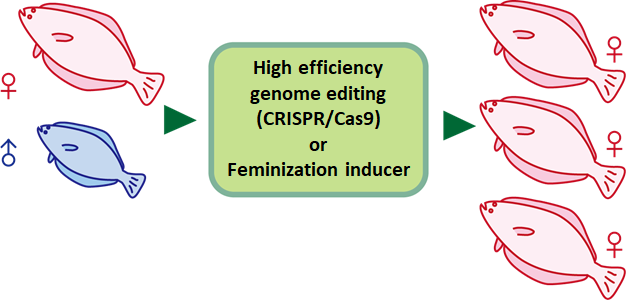
Next, we introduce the practical applications based on Prof. Kitano’s research. As mentioned earlier, it is common for males to outnumber females in aquacultures due to the high density of fishes in fish farms. This wouldn’t be a problem if male fishes are meatier than females. However for most fish species, the reverse is true that females tend to be far bulkier and economically valuable than males. This means masculinization in aquaculture is in fact not desirable for fish farmers.
To explore the practical applications of the medaka research, Prof. Kitano collaborated with the National Research Institute of Fisheries Science (NRIFS) to study flounderfarming. Similar to the medaka research introduced earlier, flounder mutants with defects in PPARα and other sex-determining genes were also isolated, in hope of engineering flounders that resist masculinization when raised in high density. Due to the consumer’s potential concerns over genetically-modified organisms, we’re also attempting to reduce PPARα expression in flounders through special feeds, as a mean to bypass genetic modifications in flounders.
In addition, Prof. Kitano is also working with Japanese freshwater eels. Female eels are typically larger than males, while having greater culinary and economic values. However, female masculinization is also a common occurrence in eel farms, and some female eels simply do not grow as large for unknown reasons. To address these problems, soy isoflavone was mixed with the eel feeds. Soy isoflavone is structurally similar to female hormones, and is expected to reduce eel masculinzation. Currently Prof. Kitano is working with eel farmers in Aichi prefecture, and preliminary results appear to be promising using this approach.

Based on these basic research, technologies that regulate sexes in fishes may have great applications in the aquaculture industries.